Understanding the Conversion from 100 g ton to ppm: A Detailed Guide
When dealing with environmental and industrial measurements, the conversion between different units is crucial. One such conversion is from 100 grams per ton (g/ton) to parts per million (ppm). This article will delve into the intricacies of this conversion, providing you with a comprehensive understanding of how to convert between these units and the significance of each.
What is 100 g ton?
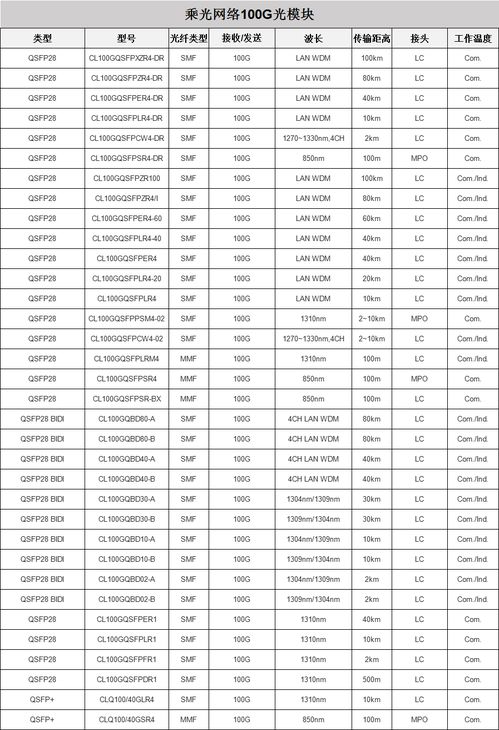
100 grams per ton is a unit of concentration commonly used in environmental monitoring and industrial processes. It represents the amount of a substance present in 100 grams of a material. For instance, if a sample of soil has a concentration of 100 g/ton of lead, it means that there are 100 grams of lead in every ton of soil.
Understanding ppm
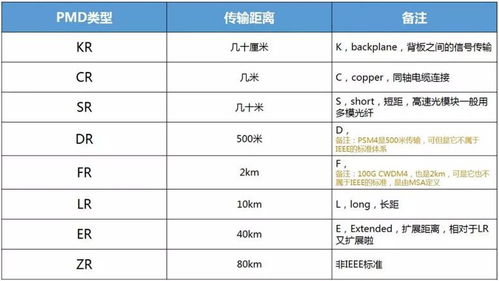
Parts per million (ppm) is another unit of concentration, often used in the same context as g/ton. It represents the number of parts of a substance present in one million parts of a mixture. In the case of lead in soil, a concentration of 100 ppm would mean that there are 100 parts of lead in every one million parts of the soil mixture.
Converting 100 g ton to ppm
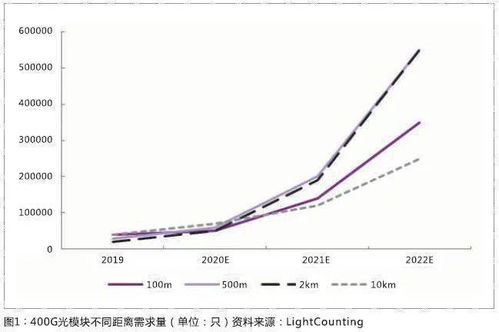
Converting from 100 g/ton to ppm requires knowledge of the density of the material being measured. The formula for the conversion is as follows:
| Formula | Explanation |
|---|---|
| ppm = (g/ton) (1,000,000 / density) | This formula takes into account the density of the material, which varies depending on the substance and its state (solid, liquid, or gas). |
For example, if you have a sample of soil with a density of 1.5 g/cm鲁 and a concentration of 100 g/ton of lead, the conversion would be:
| Input | Calculation | Output |
|---|---|---|
| 100 g/ton | 100 (1,000,000 / 1.5) | 66,666,667 ppm |
Thus, the concentration of lead in the soil sample would be 66,666,667 ppm.
Significance of the Conversion
Understanding the conversion between 100 g/ton and ppm is essential for several reasons:
-
Environmental Monitoring: In environmental studies, the conversion helps in comparing the concentration of pollutants in different samples and assessing their impact on the environment.
-
Industrial Processes: In industrial settings, the conversion is crucial for ensuring that the concentration of substances in products or waste materials meets regulatory standards.
-
Health and Safety: The conversion helps in determining the potential health risks associated with exposure to certain substances.
Conclusion
Converting from 100 g/ton to ppm is a vital process in various fields, including environmental science, industry, and public health. By understanding the conversion and its significance, you can better interpret data, make informed decisions, and contribute to the protection of the environment and the well-being of society.